Guidelines «MoveD» – Open Research Data in Swiss Movement Laboratories
This website provides information and assistance on the public sharing of research data from movement laboratories. It is structured along the data life cycle.
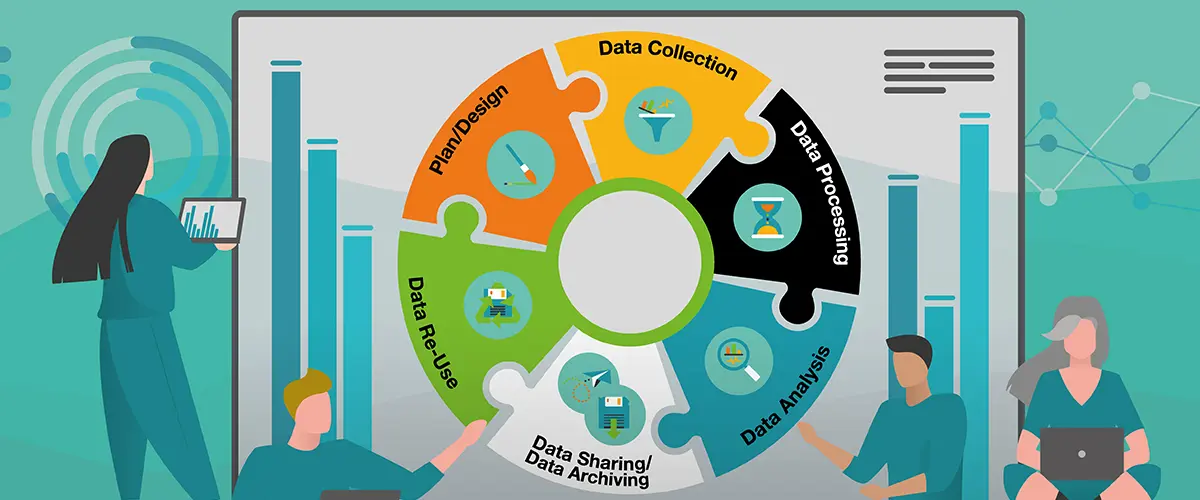
These guidelines address all the necessary steps that must be performed from planning a study up to the point where data can be published; they include ethical and legal considerations as well as data structuring processes. However, we do not aim to provide data collection standards as this is already adressed by other organizations such as ESMAC .
For each phase of the data life cycle, information that must be considered is presented. The most relevant aspects are shown on this website, while the detailed Guidelines can be found on:
Data Life Cycle
1. Plan & Design
Information is required when planning or designing a study in a movement laboratory. Several considerations have to be taken into account when the data from a project in a movement laboratory is to be shared. Please pay special attention to the following sections in the «Guidelines “MoveD” – Open Research Data in Swiss Movement Laboratories»: «Collect: Informed Consent» with reference to data sharing, «Process: Source code» on the description of codes, «Share: Licences», and «Data Re-Use: Quality Check». Staffing requirements can be found in the standards of GAMMA, Clinical Practice Recommendations from Phillips et al. (2023) or Clinical Movement Analysis Standards by Stewart et al. (2023).
2. Data Collection

Metadata that should be collected as well as marker, sensor model and informed consent considerations.
Metadata
Metadata should be collected when collecting data for each participant separately. For better reproducibility, having more personal data is beneficial. This results in ethical and legal issues, however, when sharing these data publicly. You must therefore clarify within your institution how much, and which, personal information is to be documented.
Marker / Sensor placement
When data is collected with a marker-based measurement system or body-worn sensors, it is important for the traceability and subsequent use of the data to have a clear understanding of the marker /sensor placement.
Informed consent
Swissethics provides a decision tree for finding the right template. The templates can be downloaded directly through the links provided in the decision tree. The decision tree can be found here or the German, French or Italian version here.
3. Data Processing

Metadata
Metadata that should be collected during processing and the corresponding information. If pre-processing steps have been performed during data collection, please include this information in as much detail as possible.
Source code
The mentioned requirements apply to source code.
Description of source codes/ readme files
The mentioned requirements apply to the description of source codes/readme file.
Open File Format
In this section we present recommendations as to which open data formats the files (exported from the measurement system) should be converted to. It is, however, more important to adhere to the «linked open data» framework as described here. You may be able to export your data in another file format which is not listed in the following table. Please ensure that you adhere to the «linked open data» framework mentioned above. Moreover, if additional information is generated by the measurement system, such as patient reports in the form of a PDF, for example, please add this information as well, since PDF is an open file format.
4. Data Analysis

Metadata
Metadata that should be collected during analyzing and the corresponding information. The metadata should be captured during statistical data analysis.
Sourcecode, if applicable.
The section is identical to the section «Data processing - Source code» and only applicable if the statistical software used produces code.
Description of source codes / readme file, if applicable. The section is identical to section «Data processing - Description of source codes/readme file».
5. Data Sharing

Licenses, informed consent, choosing a data repository, metadata and data. Before a licence can be issued or data shared, the copyright must be owned by the person granting the licence/sharing the data. Clarify with your supervisor or the data security officer what is applicable in your institution.
6. Data Archiving

Laws for the retention period and encoding of research data in Switzerland. Listed below are considerations that have to be made when data is archived.
Legal requirements
The laws (English, German, French) are relevant for the retention period of (research) data in Switzerland.
Key management
The laws (English, German, French) are applicable for the encoding of research data in Switzerland.
Data access
Data access should be limited to people who require access to fulfil their work within a study/project. Ideally, data access is granularly managed so that, for example, only a few people have access to the identifying information and most people on the project team only use the encoded or anonymized data.
7. Data Re-Use

Search for reuse, example repositories and how to perform a quality check.
The following methods may aid in the search for reusable datasets:
- Review relevant scholarly literature for citations and references of accessible datasets.
- Use the search engine Google Dataset Search (bear in mind that a search via Google Dataset Search does not provide conclusive results about the existence of relevant datasets as metadata determine findability).
- Search directly in a repository via the registry of research data repositories.
- Re3data provides an overview of research data repositories. By navigating through the disciplines, suitable repositories can be narrowed down and browsed through.
Templates and documents
More information on the project can be found on our project website.
Disclaimer and definitions
Legal framework
The legal framework regarding research with human beings differs in each country. As this guideline is intended to be directly applicable to Switzerland, only Swiss laws and regulations are presented. The content of these guidelines can, however, be adjusted to other countries by taking their legal framework into consideration. The user is free to do this. We do not, however, assume any responsibility regarding the interpretation of the legal text or templates for informed consent. The final decision lies with the local ethics committee.
Movement data
Movement data includes all data that is collected in a movement laboratory and takes in stereophotogrammetry, electromyography, force plates for kinetics, accelerometery, inertial measurement units, electroencephalography, plantar pressure sensors, imaging (ultrasound, magnetic resonance imaging, computer tomography), and two-dimensional video data.
Terminology
Definitions of how specific terms are used in these guidelines can be found in:
Additional information
Movement analysis
The movement laboratory allows for a deeper analysis of movement and a better understanding of motor disorders.
Research
Movement analysis
Our research units focus on issues related to health systems and health care.
The movement laboratory allows for a deeper analysis of movement and a better understanding of motor disorders.