An Enzymatic Strategy for the Asymmetric Synthesis of Ionones by Engineered Squalene-Hopene Cyclases published in “Angewandte Chemie”
Ionones are important components of the scent of flowers and fruits. The development of new synthesis routes for the production of optically pure compounds is therefore of great interest.
Ionones belong to the apocarotenoids, a family of natural products derived from carotenoids by oxidative cleavage. An efficient synthesis for these important fragrance molecules was already discovered in the late 19th century and used commercially from then on. However, the product of the so-called cation-olefin cyclisation is a racemate. Isomers differ drastically organoleptically. Therefore new synthesis routes for the production of optically pure compounds are important.
Enzymatic strategy for the asymmetric synthesis of ionones
Squalene-hopene cyclases (SHCs) are enzymes that carry out cyclisation reactions of polyene substrates with perfect stereocontrol. They accept starting molecules of varying length (C10 - C35) and can be optimised for different tasks.
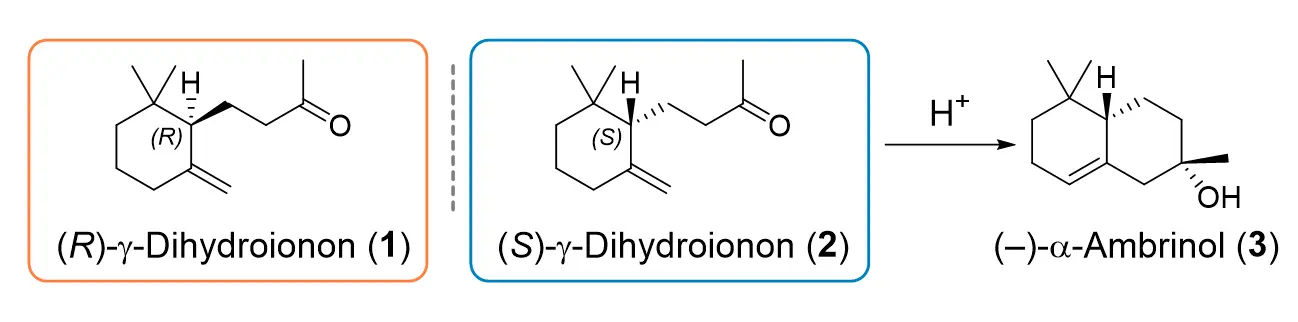
The Competence Centre for Biocatalysis together with Givaudan SA developed strategies for the use of SHCs for the asymmetric synthesis of the enantiomers of γ-dihydroionone (1, 2), which are interesting compounds for the fragrance and flavour industry. The (S)-enantiomer (2), for example, is a precursor of the compound α-ambrinol (3), which is known for its woody, tobacco and musky odour in the perfume industry.
Enzyme optimisation
In order to identify enzymes that can be used to transform the starting material (Z/E)-geranylacetone into the desired monocycle, an enzyme library of 31 natural SHC-Homologues including 18 previously undescribed enzyme variants was screened. The enzyme AciSHC from Acidothermus cellolyticus, a new SHC variant, produced in whole-cell biocatalyses in addition to a bicyclic product a small amount of the desired monocyclic product (R)-γ-dihydroionone (1). By means of directed evolution, the production of production of (1) could be significantly improved.
By analyses of the pure, geometric isomers nerylacetone (Z) and geranylacetone (E), it was shown that the showed that optimised variants of the enzyme AciSHC in dependence on the spatial orientation of the starting substrate produced either the desired monocyclic compound or a bicyclic product.
Substrate strategy
The geometry of the substrate was also important for the synthesis of the enantio-complementary (S)-γ-dihydroionone (2) by the industrially relevant SHC variant AacSHC_215G. Based on the findings on the production of (R)-γ-dihydroionone, the carbonyl group of the (E)-isomer was chemically protected, in order to prevent the formation of the bicyclic ring. Through this substrate engineering, a monocycle was produced, which can be chemically converted into the desired (S)-enantiomer (>99 % ee).
Mechanism
The study shows that the stereoselectivity of the enzyme-catalysed cyclisation and the subsequent termination of the cascade depends on the double-bond geometry of the starting material. This principle can be applied to the asymmetric synthesis of further cyclic natural products and is a powerful new tool for the fast and highly efficient assembly of complex carbon skeletons.
Reference:
Asymmetric Cation-Olefin Monocyclization by Engineered Squalene-Hopene Cyclases
M. Eichenberger, S. Hüppi, D. Patsch, N. Aeberli, R. Berweger, S. Dossenbach, E. Eichhorn, F. Flachsmann, L. Hortencio, F. Voirol, S. Vollenweider, U. Bornscheuer, R. Buller. Angewandte Chemie. 04 August 4, 2021. https://doi.org/10.1002/anie.202108037