Cell Cultivation Techniques, Tissue Engineering, and Medical Biology

«In the future, cell cultivation techniques will become increasingly important beyond the pharmaceutical sector, as it also supports sustainable agriculture without the need for plants and animals.»
Prof. Dr. Ing. Regine Eibl-Schindler, Head of section
Competencies, Infrastructure, and Equipment
The Cell Cultivation Techniques Group deals with processes involving plant, animal, and human cells (including human mesenchymal and induced pluripotent stem cells). Cell biology, biochemical engineering, and process technology expertise are used to quickly develop processes that provide products for the pharmaceutical, cosmetic, and food industries from lab to pilot scale. The group's activities include teaching, research/services, and continuing education.
For experimental work, the Cell Cultivation Techniques Group has modern technical equipment for establishing and cultivating cell lines. This includes sterile workbenches, incubators, mixers, storage tanks, stirred, shaken, and wave-mixed bioreactors with working volumes up to 200 L. In addition, the group also has access to equipment for downstream processing, automated devices for cell counting, and for monitoring substrates, metabolites, and protein-based products, flow cytometry instruments for monitoring cell quality, and ATF systems for implementing intensified production processes. Noteworthy is our significant share in single-use technology, which is increasingly implemented in industrial settings. Additionally, specialized analytical equipment like HPLC and MS devices from the ICBT and the expertise of specialists there can be accessed if needed.
Continuing Education
The Cell Cultivation Techniques Group offers tailored training courses on cell cultivation for companies. It has also resulted in the development of an edX course (Cell cultivation techniques for beginners:https://www.edx.org/course/cell-cultivation-techniques-an-introduction). The course is designed for those with both biological and technical backgrounds and consists of 4 parts: (1) Basics of biochemical engineering for cell cultivation, (2) Mammalian cell cultivation and antibody production, (3) Stem cell expansion, (4) Cultivation of plant cell cultures.
Sample Projects
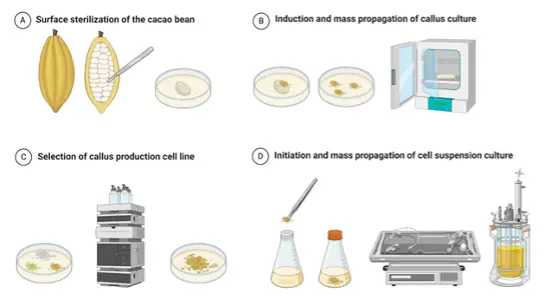
The World's First Cell Culture Chocolate (Department N's seed funding project)
In autumn 2017, the world's first cell culture chocolate was produced in the laboratories of the ILGI and ICBT. To achieve this, cocoa suspension cells, propagated in a wave-mixed bioreactor, were freeze-dried, harvested, and roasted. The resulting cocoa powder was then processed into dark chocolate by adding sugar and cocoa butter. At the time, this Proof-of-Concept process provided 3 chocolate bars (10 L culture broth). The cell culture was derived from a callus culture of cocoa beans and maintained in a food-grade MS medium. Remarkably, the cell cultures used produced comparable amounts of chocolate polyphenols (epicatechin, procyanidins, cinnamtannins) to those found in conventional cocoa beans. Subsequent studies focused on optimizing and scaling-up the process to pilot scale (max. 100 L working volume). It was found that the secondary metabolite profile and thus the flavor profile and taste of the produced cell culture chocolate could be influenced by the type of bioreactor (EP 4 176 710 A1). While the cocoa cell biomass produced in the wave-mixed or orbitally shaken bioreactors produced a very fruity note, the stirred bioreactor cultivations resulted in a malty flavor, and the pneumatically driven bioreactor cultivations produced a floral note.
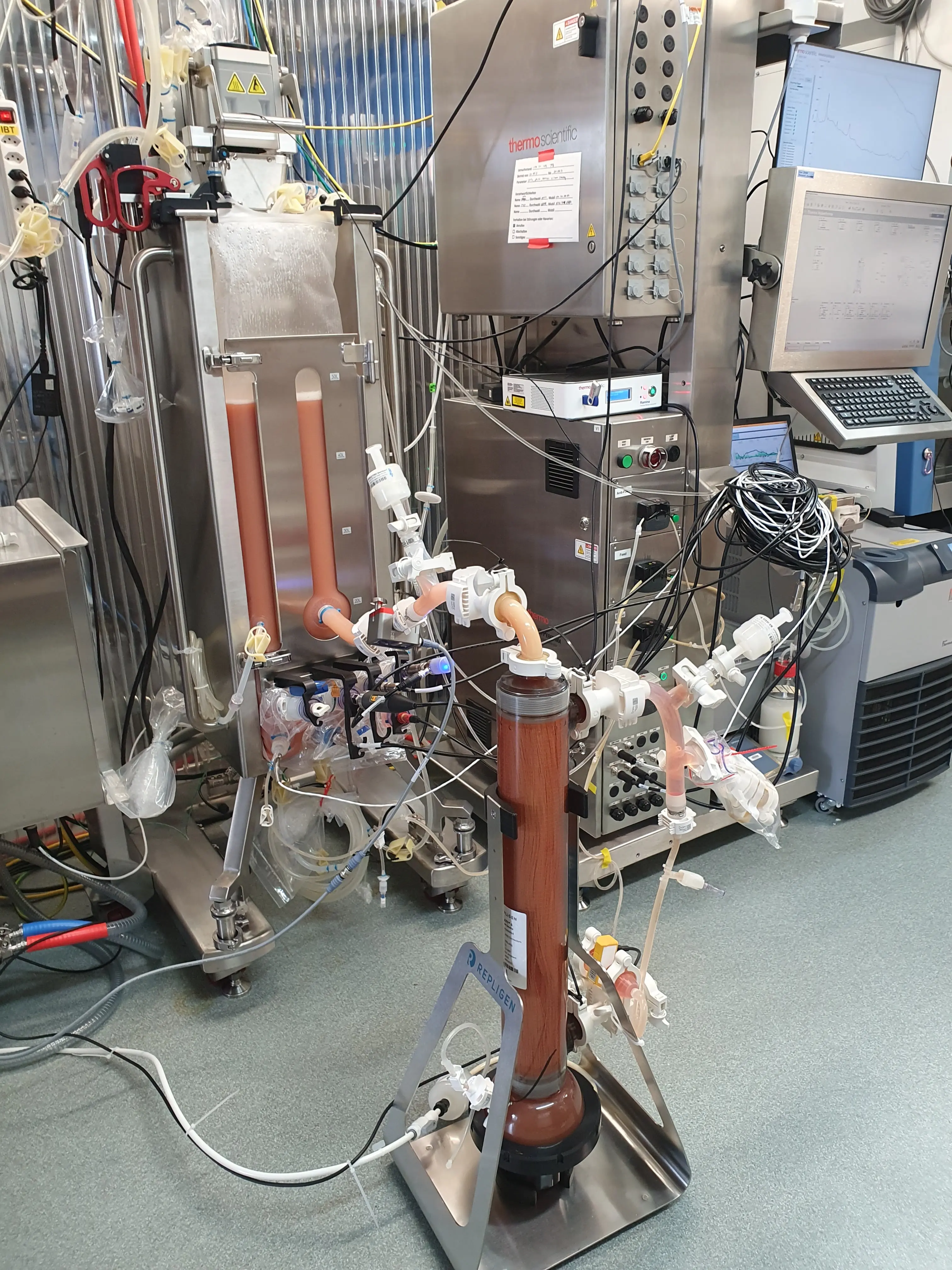
Intensified and Continuous CHO-Based IgG Productions in HyPerforma DynaDrive S.U.B. 50 L (Research project funded by ThermoFisher Scientific)
Monoclonal antibodies (mAbs) are Immunoglobulin G (IgG) molecules that are among the fastest-growing products in the pharmaceutical industry. They are primarily produced using genetically modified Chinese hamster ovary (CHO) suspension cells. To reduce development and production costs for mAbs, process intensification approaches have come into focus for many mAb developers and producers over the past 10 years, particularly in upstream processing (perfusion processes) combined with the implementation of single-use technology.
The HyPerforma DynaDrive S.U.B. is the first single-use bioreactor specifically developed for intensified processes. The bioreactor design constitutes a cubic bag, containing a ladder-like impeller and a sparger. The bioreactor offers excellent mass transfer at low shear forces and without the need for baffles. At specific power inputs of 80 W m⁻³, mixing times of less than 60 s can be achieved, with kLa values of up to 50 h⁻¹. Given the DynaDrive’s high turn-down ratio (difference between the maximum and minimum working volume), our studies showed that it was possible to directly inoculate the bioreactor with a single vial of an ultra-high cell density (UHCD) working cell bank when using the 50 L version. In the subsequent low-seed fed-batch processes using ExpiCHO-S cells (Gibco) and Efficient-Pro medium (Gibco), as well as Efficient-Pro Feed 2 medium (Gibco), maximum concentrations of 3.58 g L⁻¹ of IgG could be achieved in 19 days. As such, the maximum achievable IgG concentration and product quality were comparable to those reached using conventional production methods. However, by using the cells from the UHCD working cell bank to inoculate the bioreactor, the process time could be shortened by almost 60 % [1].
Furthermore, the continuous operation of the DynaDrive S.U.B. over 50 d (max. 1 vvd) using a CHO K1 cell line (product: Trastuzumab) and Gibco's high-intensity perfusion CHO medium permitted volumetric productivities of greater than 1 g L⁻¹ d⁻¹ to be achieved. Such productivities are 3 times higher than those routinely found when pursuing a low-seed fed-batch process with Efficient-Pro Feed 1 medium (Gibco) in the HyPerforma DynaDrive 50 L [2-3].
Development of a Degradable/Implantable Microcarrier for Human Mesenchymal Adipose Stem Cells Used in Cell Therapies (Innosuisse Project: 25275.2 PFLS-LS)
Human mesenchymal adipose stem cells (hASCs) are recognized for their great potential in the production of cell therapeutics. There is interest in allogeneic therapeutic approaches, which require large quantities of clinical-grade hASCs. This necessitates efficient cell expansion methods, with the most promising results achieved when using microcarrier operated-stirred bioreactors.
Within the scope of this project, a degradable/implantable microcarrier (BR44) for hASC expansion in stirred cultivation systems was developed by MicroSphere and qualified by Cardiocentro Lugano and the ZHAW working group. This was conducted under chemically defined conditions, considering biophysico-chemical, cell biological, and culture technical aspects. Alongside an immortalized model cell line, primary patient cells were also used in the cultivations (Ursuppe medium) in T-flasks and spinner flasks. Details to the project may be found in the publications by Muoio et al. [4-5].
[1] J. Ott, V. Ott, P. Neubauer, D. Eibl, R. Eibl (2024) Process intensification by inoculating antibody production bioreactors directly from cryovials. Chem. Ing. Tech. 96(4), 453-461. DOI:10.1002/cite.2023000189
[2] V. Ott, J. Ott, D. Eibl, R. Eibl (2024) Scaling fed-batch and perfusion antibody production processes in geometrically dissimilar stirred bioreactors. Processes 12(4), 806. https://doi.org/10.3390/pr12040806
[3] M. Bates, K. Broadbelt, J. Ott, V. Ott, R. Eibl, L. Chen, D. Kuntz, L. Zhang, M. Zustiak, S. Woods (2024) Using Raman Spectroscopy as a Process Analytical Technology Tool in a 50-Day Continuous Perfusion Run. https://assets.thermofisher.com/TFS-Assets/CAD/Application-Notes/raman- spectroscopy-process-analytical-technology-tool-an1068-en.pdf
[4] F. Muoio, S. Panella, M. Lindner, V. Jossen, Y. Harder, T. Moccetti, R. Eibl, M. Müller, T. Tallone (2021) Development of a biodegradable microcarrier for the cultivation of human adipose stem sells (hASCs) with a defined xeno- and serum-free medium. Applied Sciences 11(3), 925.
[5] F. Muoio, S. Panella, V. Jossen, M. Lindner, Y. Harder, M. Müller, R. Eibl, T. Tallone (2021) Human adipose stem cells (hASCs) grown on biodegradable microcarriers in serum- and xeno-free medium preserve their undifferentiated status. Journal Functional. Biomaterials 12: 25. https:// doi.org/10.3390/jfb12020025
Staff
-

Prof. Dr. Ing. Regine Eibl-Schindler
ZHAW School of Life Sciences and Facility Management
Center for Cell Cultivation Techniques, Tissue Engineering and Medical Biology
Grüentalstrasse 14
8820 Wädenswil -

ZHAW School of Life Sciences and Facility Management
Section for Cell Cultivation Techniques
Grüentalstrasse 14
8820 Wädenswil -

ZHAW School of Life Sciences and Facility Management
Section for Cell Cultivation Techniques
Grüentalstrasse 14
8820 Wädenswil -

ZHAW School of Life Sciences and Facility Management
Section for Cell Cultivation Techniques
Grüentalstrasse 14
8820 Wädenswil -

ZHAW School of Life Sciences and Facility Management
Section for Cell Cultivation Techniques
Grüentalstrasse 14
8820 Wädenswil -

ZHAW School of Life Sciences and Facility Management
Section for Cell Cultivation Techniques
Grüentalstrasse 14
8820 Wädenswil -
ZHAW School of Life Sciences and Facility Management
Section for Cell Cultivation Techniques
Grüentalstrasse 14
8820 Wädenswil -

ZHAW School of Life Sciences and Facility Management
Section for Cell Cultivation Techniques
Grüentalstrasse 14
8820 Wädenswil -

ZHAW School of Life Sciences and Facility Management
Section for Cell Cultivation Techniques
Grüentalstrasse 14
8820 Wädenswil