Micelle Formation in Polymer / Copolymer Blends
In this project a blend of amphiphilic block copolymer (a copolymer with a hydrophilic and a hydrophobic block) and hydrophobic homopolymer was analyzed. The goal was to predict the critical micelle concentration (CMC) as a function of temperature, polymer length and copolymer composition.
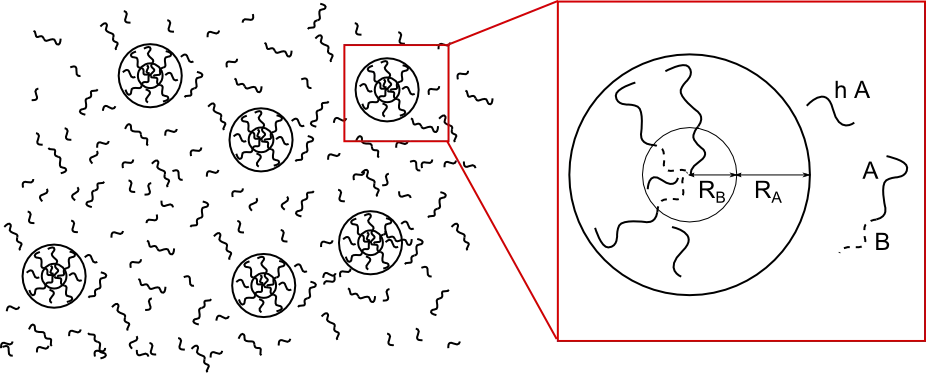
Similar to surfactants dissolved in e.g. water, the block copolymers A-b-B can self-assemble into micelles with the homopolymer hA acting as a solvent. To reduce the unfavorable interaction with hA the hydrophilic blocks accumulate in the micelle core whereas the hydrophobic blocks stick out to build the corona.
To form micelles a certain minimum surfactant or block copolymer concentration is needed. For low copolymer concentrations the polymer / copolymer blend builds a uniform mixture. If the copolymer concentration is increased, at a certain concentration the number of micelles rises abruptly. This is the critical micelle concentration (CMC).
Assuming spherical micelles, we used a scaling ansatz (e.g. de Gennes 1978, Leibler etal. 1983) to compute the free energy of the coploymer / homopolymer blend as a function of temperature, number of micelles and so on. The equilibrium configuration of the system, and thus the CMC, was then determined as the minimum of the free energy.