Proton Exchange Membrane Fuel Cell
A proton exchange membrane fuel cell (PEMFC) is an electrochemical energy conversion device. It converts chemical energy stored in a fuel to electrical energy. Typically, hydrogen is used as a fuel in a low temperature fuel cell. Fuel cells are designed for continuous replenishment of the fuel consumed, so fuel cells differ from batteries in that way. This type of fuel cell is currently being developed intensively; fields of application are for transportation, back-up power, portable power and small distributed generation.
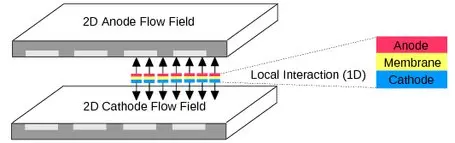
PEMFCs use a platinum coated solid polymer to produce electricity from hydrogen and oxygen. PEM fuel cells have a high power density and are low in weight. They are also able to operate in low temperatures, typically around 80ºC which allows them to start quickly.
Basically, the physical structure of a PEMFC consists the following components: gas distribution channels, gas diffusion layers, the catalytic layers in the anode and the cathode, and the polymer membrane. The PEMFC combines in a very compact unit the electrodes and the electrolyte. This structure, well known as membrane electrode assembly (MEA), is not thicker than a few hundred microns. It is the heart of the fuel cell and is fed with hydrogen and oxygen, generating electrical power with a power density of around 1 W cm−2.
The polymeric solid electrolyte forms a thin electronic insulator and a barrier for gases between both electrodes, allowing fast proton transport and high current. The solid electrolyte has the advantage, as opposed to those of liquid type, that allows the FC to operate in any spatial position.The porous Pt/C electrodes are permeable to gases. They consist of a catalytic layer of great superficial area. Electrocatalyst materials are necessary to obtain a good operation, increasing the speed of the chemical reaction. In this way, the gases can react with a lower energy of activation, allowing the reaction to take place at a lower temperature.
Modeling of PEMFCs
Mathematical modeling and simulation can significantly advance the development and optimization of proton exchange membrane fuel cells. Multiphysics (or coupled) models represent the interaction between the transport and reaction processes that are present in an operating fuel cell. Analyzing these interactions by combination of simulation and measurement is essential to identify the energy conversion losses and to improve the fuel cell performance.
Challenges facing developers of proton exchange membrane fuel cells include:
- the interpretation of experimental data taken from a fuel cell test stand (single cell or stack)
- Identification of model parameters from experimental data
- Accurate prediction of spatial distribution of the current and temperature
- Water management
- Operational issues such as temperature dynamics on change in power demand, optimization of start-up, etc.
- Control system design
- Analysis of cell degradation
- Optimization of the platinum usage
PEMFC Modeling at the ICP
At the Institute of Computational Physics we have been developing models accounting for all important processes and couplings that govern the behaviour of fuel cells and fuel cell systems. Largely different length and time scales must be considered. Our modelling approaches for proton exchange membrane fuel cells address three different scales that are important to describe the transport processes:
- modelling of the membrane electrode assembly
- single cell models
- stack models